Titanium dioxide is also called titania which occurs oxide of titanium in nature, and its resources are ilmenite, rutile, and anatase. It is a large-band gap semi-conductor. In , world production exceeded nine million metric tons, and it has been valued at $13.2 billion. Since it has unique optical and chemical properties, titanium dioxide nanoparticles have been studied and numerous applications are found. They are also called ultrafine titanium dioxide. Small size add a significant property to nanoparticles of titanium oxide which makes it photocatalytic material. High chemical stability, and strong oxidizing power are also properties of titanium dioxide nanoparticles among others.
Read more
Figure: In the rutile titanium dioxide crystalline structure, titanium and oxygen atoms are shown as grey and red spheres respectively.
G. Narejo, and W. F. Perger, First Principles Computations of Second-Order Elastic Constants (SOEC) and Equations of state of Rutile TiO2, .
In consideration of being a rutile titanium dioxide nanoparticles as you can see the structure in the figure above, the nanoparticle one is more absorbent than larger size, and it has many synthesizing methods like Laser Ablation method. Since the band gap of rutile titanium dioxide nanoparticles is 3 eV which is lower than band gap of zinc oxide, and it has already applications in semiconductor technology as sensors. Therefore, rutile titanium dioxide nanoparticles are commonly studied in photonics, and semiconductor technology. It can be functionalized to absorb UV radiation in commercial applications. It can be additive or component of composites to insert photocatalytic activity to composites. One can also mix rutile and anatese to make band gap alignment.
A research group at Changwon National University conducted a study that they synthesized rutile titanium dioxide nanoparticles using sol-gel method followed by hydrolysis of TiCl4 at low temperature. They also found that the size is 26.4 nm at 300 C°, and the size increase with temperature. Another research group studied photocatalytic applications of room temperature rutile titanium dioxide nanoparticles, and they concluded that it shows a remarkable photocatalytic efficiency on degradation of MB Dye, RB Dye, and PNP. They examined the degradation of materials via UV-Vis spectrophotometry. As a result, degradations of MB Dye, RB Dye, and PNP are 97%, 98%, and 80% respectively.
Rutile titanium dioxide nanoparticles offer tremendous applications in many fields. There are researchers conducting experiments to develop new and cost effective synthesis methods, and many more applications will be found.
Monthly Tech-Tip from Tony Hansen SignUp
No tracking! No ads!
Titanium Dioxide
Alternate Names: TiO2
Description: Anatase, Brookite
Notes
TiO2 occurs in many silicates in nature, accounting for over 1% of the earth's crust. Thus it is manufactured using a variety of materials and processes. Titanium dioxide power is very fine-grained and it agglomerates, so glazes containing it need to be sieved to break down the small lumps (even a high-speed propeller mixer often won't do it).
Although titanium is the strongest white pigment known for many uses, in ceramics the whiteness (and opacity) it imparts to glazes is due to its tendency to crystallize during cooling. While titanium dioxide is used in glazes as an opacifier, it is not as effective and easy to use as tin oxide or zircon. It can be used as an additive to enliven (variegate, crystallize) the color and texture of glazes by introducing crystallization. Rutile works in a similar manner, typically both become saturated in the melt beyond about 5-6%, producing a dry and unstable glaze surface. In moderate amounts, it encourages strong melts, durable surfaces and rich visual textures.
Titanium is available both as raw and surface-treated products. Non-pigmentary grades flow more freely in the dry state. Self-opacified enamels are made by adding titanium during smelting to supersaturation. Upon firing the enamel, the titanium crystallizes or precipitates to produce the opacity. Titania is also used in dry process enameling on cast iron appliances for its effect on acid resistance, color and texture. In glass, non-pigmentary titanium dioxide increases the refractive index and intensifies color.
Related Information
Original Container Bag of Titanium Dioxide
Additional resources:What is PDMS silicone oil used for?
4 Tips for Selecting the Perfect P2NP4 Tips for Selecting Bulk Nano Barium Sulphate PowderChuangge contains other products and information you need, so please check it out.
Variegating effect of sprayed-on layer of titanium dioxide
The base glaze (inside and out) is GA6-D Alberta Slip glaze fired at cone 6 on a buff stoneware. However, on the outside the dried-on glaze was over-sprayed with a very thin layer of titanium and water (VeeGum can be used to help gel the sprayable titanium slurry and suspend it). The dramatic effect is a real testament to the variegating power of TiO2. An advantage of this technique is the source: Titanium dioxide. It is a more consistent source of TiO2 than the often-troublesome rutile. Another advantage is that the variegation can be selectively applied in specific areas or as a design. This effect should work on most glossy glazes having adequate melt fluidity.
Thin titanium band sprayed over cone 6 glazes demonstrates crystallization
The first is on GA6-A, the rest are on GA6-C (Alberta slip glazes). The last has been applied too thickly, the brown band is dry and blistered.
Titanium as an opacifier
This is a lithium glaze fired at cone 6. It has 6% titanium. This effect will also work in other types of transparent recipes. There should be more blue with slower cooling. Mixing some rutile in (e.g. 4 titanium, 2 rutile) should enhance also. 6% is pushing the edge of how much titanium should be in a recipe. Any more, or cooling too slow, could transform the surface into a mass of white crystals (which would be rough and non-functional). It is best to manually program firings, the up and down schedules? That would make the result consistent no matter how heavily or lightly loaded a kiln is.
Titanium Dioxide in a cone 6 calcium matte glaze
The glaze is GZ1 cone 6 base calcium matte on Plainsman M390 fired at cone 6 using the PLC6DS schedule. 5% titanium dioxide has been added. Titanium can create reactive glazes, like rutile, with no other colorants added. This effect also works well on matte surfaces, but the glaze needs good melt fluidity (that is good because functional mattes melt well). Calcium mattes host crystallization and work particularly well. Because titanium dioxide does not contain iron oxide lighter colors and better blues are possible compared to rutile (iron is still needed by it is coming from the body here). Like rutile, the effects are dependent on the cooling rate of the firing, slower cools produce more reactivity. Even application without drips is important (mixing as a thixotropic dipping glaze is best). This appearance also depends on using dark burning body or engobe.
The titanium/stain mechanism at cone 10R
These are porcelain and stoneware mugs. The glaze is GD (based on the fritted GB version of GA). This project started with calculations to source boron from a frit (instead of Gerstley Borate), MgO from talc and a frit (instead of dolomite). These moves enabled eliminating raw silica from the recipe. This produced a finer silky texture and better melt fluidity (for hosting colors). Adding rutile and zircopax produced a great bamboo. But what about variegating using titanium instead of rutile (it contains no iron so colors should be brighter)? Jackpot with this red stain! The titanium has done it's job even a little too well. Cobalt and titanium also worked.
A titanium/colorant addition to a cone 10 magnesia matte glaze
These porcelain mugs have the same glaze, the one on the left was fired at cone 10R (gas), the other at cone 10 oxidation (electric). This is our standard cone 10R magnesia matte, GB. We have added a 5% Mason encapsulated red stain and 4% titanium dioxide (producing recipe code number GD). While the reduction version looked good the oxidation one turned out much more vibrant. And it feels much better, being very pleasant to touch. The marbling is a bit excessive so in GD1 we reduced the titanium by 1% (and increased the stain by 1%). MgO matte base recipes are very receptive to this type of adjustment and they work across a wide range (from low to high temperatures). Titanium is much better for variegating bright colored glazes than rutile, because the latter contains lots of iron that muddies the color.
A titanium/colorant addition to a cone 6 magnesia matte glaze
This is the GA recipe (a 90:10 mix of G and GB), it normally produces a silky matte if not cooled too quickly. Shown on the left is our original addition of 8% Mason red stain and 4.5% titanium dioxide. This not only did not produce the desired marbled effect, it actually made it more glossy! A 1.5% titanium addition completely transforms it to what you see on the right. Rutile, as a source of TiO2, is often used for this, but it is high in iron and would completely muddy the red color. Pure titanium dioxide, by contrast, is iron free.
Titanium instead of rutile for floating blue
Rutile blue glazes are actually titanium blues (because rutile mineral is an impure source of TiO2 and Fe2O3). The iron and titanium in the rutile react to form the floating blue effect. The GA6-C recipe has always relied on a 4% rutile addition. Its GA6-A base recipe contains significant iron (because of the 80% Alberta Slip), so could titanium oxide deliver the same floating blue effect? Yes.
These mugs are M390 clay. The top left one is the standard GA6-C (with rutile) fired using the C6DHSC slow-cool firing schedule (the bottom left normal cool PLC6DS schedule produces little color). But the ones on the right switch the 4% rutile for titanium dioxide (the L recipe). The top right was fired using the slow cool, the bottom right was the normal cool schedule.
Titanium is a much more consistent and reliable material than rutile. If it can produce an excellent blue color is produced even without a slow cool (lower right) then it is a better long-range choice.
Same glaze, same firing - titanium accounts for the difference
The glaze is L fired at cone 6 using the C6DHSC firing schedule (the glaze is Alberta Slip with a frit addition and 4% titanium dioxide). These mugs were in the same firing. On the porcelain (left) the glaze fires the expected floating blue. The degree of difference on the right has two contributing apparent factors. While other clay bodies of similar color do not affect this glaze as much, the body used in the mug on the right contains Plainsman 3B, at cone 6 it vitrifies (releasing iron compounds) and it releases iron in soluble salts that are interacting with the glaze. Titanium is very sensitive to the presence of iron and this body is making it available in an effective form. The difference would be much less if rutile had been used, since it already contains significant iron.
Zircopax, tin oxide, titanium as opacifiers in four base glazes
The body is Plainsman M390. Firing is the cone 6 PLC6DS schedule. Each horizontal row is a commonly-used base glaze. The top one is an MgO matte, the next one down is a calcium matte, row 3 is GB glossy and row 4 is Ravenscrag Slip+frit. The two mattes behave very differently from each other with the additions of opacifier. Thickly applying an opacified glaze will obviously affect visual character (column 4). Tin oxide fires whiter than zircon (e.g. Zircopax). If you like the G recipe, consider the GY variant for better melting.
A hazard of using titanium opacifier in a glaze
On a clay test tile this titanium opacified cone 6 oxidation glaze, GZ1, looks great. But it is important to recognize that its variegated fired appearance is a combination multiple factors: The chemistry of the glaze, the titanium, quality laydown, the PLC6DS firing schedule, the red M390 clay body and variations in the thickness-of-application. However, the last four of those factors changed with the mug on the right! It is made from buff-burning M340! There are drips from uneven drainage during glazing. The slow cool C6DHSC firing schedule. Notice how it is actually going transparent where very thick. An even laydown was not achieved since the slurry was not properly mixed, it contains calcined kaolin and requires special attention to achieve a thixotropy.
GC rutile blue on P700 at cone 10R
The clay body is Plainsman P700. This was fired in cone 10R using the C10RPL firing schedule. The outside glaze is GC. The inside glaze is GU.
The surprising results of titanium/talc additions to Ravenscrag slip at cone 10R
Talc additions to RCS produce variegated silky matte surfaces (in 10-15% amounts) at cone 10R. What if titanium is also added? This triaxial blend shows what works.
Top tile #1: Pure Ravenscrag Slip (RCS)
Bottom left tile #7: 85% RCS, 15% Talc.
Bottom right tile #10: 95% RCS, 5% Titanium Dioxide. The addition of a little titanium further improves the effect as shown on #8 (88% RCS, 10% Talc, 2% Titanium) and #6 (90% RCS, 10% Talc, 3.5% Titanium). But too much titanium loses it (tile #9) and adding only titanium goes glossy brown. This shows that some TiO2 is good, too much and the effect is lost.
For more Rutile Type Titanium Dioxideinformation, please contact us. We will provide professional answers.
Links
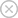



Comments
All Comments ( 0 )